
Advanced Data Logging Solutions for Life Sciences and Healthcare
Ensure Product Integrity and Safety with Precise Environmental Monitoring from Lascar Electronics
Simplify Temperature Compliance
Lascar’s EasyLog solutions, tailored for the life sciences and healthcare sectors, offer cutting-edge data logging technology designed to monitor critical environmental variables that can significantly influence operational efficiency, product integrity, and safety. Temperature, humidity, CO₂ levels, and air quality have a significant impact on workplace safety and the quality of sensitive items such as vaccinations, medications, and food.
Lascar Electronics is at the forefront of environmental monitoring, offering some of the most advanced solutions available on the market today. By integrating innovative data logging devices, organizations can actively monitor and manage the environmental conditions that are crucial for maintaining the efficacy, stability, and safety of their products.
These solutions not only facilitate compliance with global standards and regulations but also empower businesses to optimize their operations, reduce waste, and enhance product quality. Lascar’s technology delivers the accuracy and peace of mind that professionals in life sciences and healthcare demand.
Trusted by Global Organisations

Applications
With hundreds of data loggers and sensors offering a variety of connectivity types and measurement parameters (and over a million devices in the field), Lascar’s EasyLog products cater for a wide variety of applications.




Lascar’s Data Logging Process is Fast, Precise and User Friendly

Wireless, USB, Bluetooth and SMS connectivity for comprehensive and wireless monitoring.

Temperature measurement ranges from -200°C up to 1350°C (-328°F / 2462°F).

Configurable alarm levels with onboard warning light and sounder.

No need to remove it from service for recalibration, just replace the probe.

Wirelessly stream and view data on the EasyLog Cloud.

21CFR Part 11, using encrypted, non-editable formats with our 21CFR Cloud.

Device memory stores all data even if WiFi is temporarily disconnected.

Traceable calibration certificate supplied at 2°C and 8°C.
Featured Products
-
Temperature Data Loggers$107.50 ex. taxAdd to cart
- Temperature Data Logger
- -35 to +80°C
- High contrast LCD display
- Stores over 16,000 readings
- Configure and download data via USB
- Programmable alarm thresholds
-
Temperature & Humidity$207.42 ex. taxAdd to cart
- Temperature & RH Logger
- -20 to +60°C / 0 to 100%RH
- Large format LCD display
- Connects to EasyLog Cloud via Wi-Fi
- Programmable alarm thresholds
- Email and SMS notifications
-
Vaccine Monitoring$376.30 ex. taxAdd to cart
- High-Accuracy Vaccine Monitor
- -40 to +60°C
- Onboard Light and Sound Alert
- Calibratable Probe
- EasyLog Cloud Connected
-
Air Quality
Air Quality, Carbon Dioxide, Particulates, Pressure, Temperature & Humidity, VOCs
$380.79 ex. taxAdd to cart- Indoor Air Quality Monitor
- Connected to the EasyLog Cloud
- Audible and Visual Alerts
- Coloured Status Ring
- Internal back-up power
-
Temperature Data Loggers$546.54 ex. taxHigh-Accuracy WiFi Dual Channel Cryogenic Vaccine Data Logger with DisplayAdd to cart
-
Vaccine Monitoring
Learn More About Lascar Electronics
The Importance of Temperature Measurement for Vaccine Storage
The potency of vaccines degrades quickly when they aren’t stored within the correct temperature range. Monitoring and recording temperatures within refrigerators, freezers and containers used for storing vaccines is therefore a well-established best practice. However, ensuring that readings taken match the…

Temperature Monitoring Deep Frozen Vaccines
Many vaccines contain living cells, and so when stored for any length of time, they must be kept within a suitable temperature range to prevent degradation. Vaccine shots that are allowed to fall outside the safe storage range quickly lose their potency, meaning patients may be poorly immunized or even gain…

WiFi Data Loggers Save 900 COVID Vaccines From Going to Waste
Safe vaccine storage is essential if you want to maintain sample quality from the time of manufacture to the point of administration. To achieve this, vaccines must be transported, handled and stored within an appropriate temperature range of 2°C – 8°C. Once temperatures exceed these limits…
Cloud-Based Monitoring
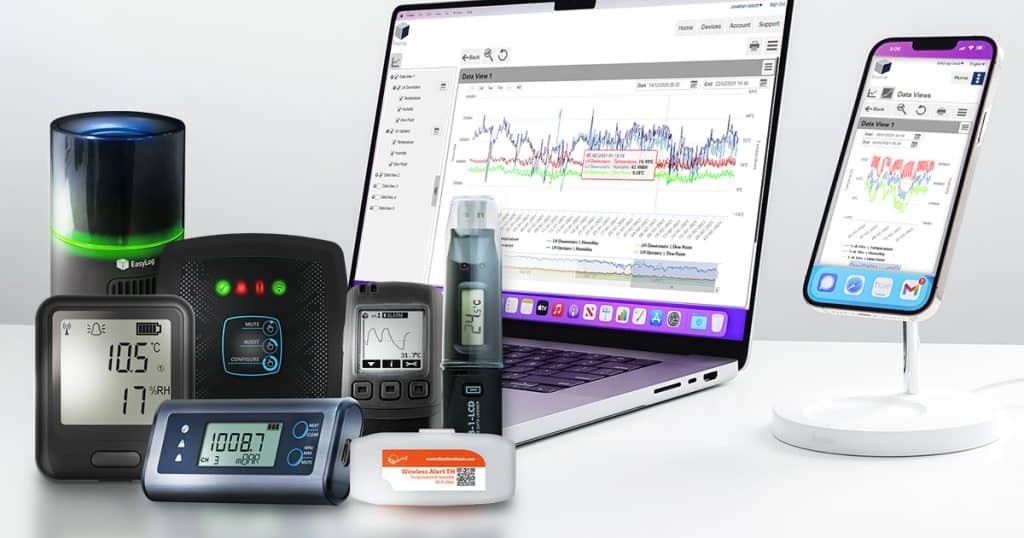
Real-time data access and analysis is not only beneficial but also necessary. With its smooth, safe, and scalable solutions, EasyLog Cloud delivers a major improvement in data management and monitoring, enabling you to stay connected to your data at all times and locations.
21CFR Part 11
21CFR is a set of regulations in the US Food and Drug Administration (FDA). Part 11 provides the criteria for the FDA to accept electronic records as equivalent to signed paper records with regard to data security. Even outside the USA, 21CFR Part 11 compliance is fast becoming the de facto global standard for electronic records management and control in the food and pharmaceutical industries.
21CFR compliance is required for US food, pharmaceutical and healthcare companies and any such companies outside the USA that want to operate in the USA.
EasyLog 21CFR-Compliant data loggers include the following special features:
Ability to create users and assign individual users with specific permissions.

Full software and session data audit trails showing user activity.

All reports show who has started, stopped and approved the session data.

Encrypted data storage, which cannot be edited.

Email alerts sent for failed login attempts.

Comment approval by an authorised user for final submission.

SPEAK TO AN EXPERT
Reach out to a Lascar professional today for a personalized consultation.

STAY UPDATED
Join our mailing list for the latest Lascar updates, and news and receive our exclusive offers.








