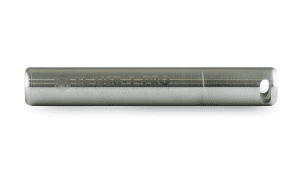
This 21CFR Part 11 compliant, standalone, vaccine monitoring data logger records and displays over 32,000 temperature readings from the supplied high-accuracy glycol bottle thermistor probe. The data logger features an LCD and push-button which allow the user to cycle through the most recent, highest and lowest stored readings.
Why buy a 21CFR logger?
The 21 CFR Part 11 regulations issued by the US Food and Drug Administration (FDA) give the criteria under which electronic records can be considered by the FDA as equivalent to paper records. If you need to meet these regulations or have enhanced data security requirements then you should choose one of our 21CFR loggers, otherwise our standard loggers will suffice. Learn more about 21 CFR Part 11.
Used in conjunction with Lascar’s 21CFR Part 11 software, the logger enjoys all of the core offerings of the original EasyLog software, but with additional 21CFR characteristics including user permissions, encrypted data which cannot be edited, full audit trail and electronic signatures for all activity.
Easily set up the logger and view downloaded data by plugging the unit into a PC’s USB port and using the free 21CFR Part 11 software provided. Supplied with a long life ½ AA lithium battery, wall mount clip, glycol bottle thermistor probe and traceable calibration certificate.
This product meets and exceeds CDC standards for vaccine monitoring.
This product complies with BS EN 12830:2018 (Temperature recorders for the transport, storage, and distribution of temperature-sensitive goods).
PLEASE NOTE: You can add up to TWO SPARE BATTERIES for every data logger ordered. If you wish to order more than this quantity, please contact your local sales office for special shipping information.
Calibration testing carried out on our products provides assurance of the ongoing accuracy of your EasyLog data logger. This is relevant for all users, but is especially important for medical, food and scientific applications, and where audit compliance needs to be demonstrated. We provide a number of standard calibration options suitable for common applications such as monitoring fridges, chilled goods and freezers, but can also provide customized calibration at whatever measurement points you need. Please contact our sales team for details.
The EL-21CFR-VAC comes with a calibration certificate issued at 2 and 8°C. It typically takes around 2 weeks (Lascar UK and Asia customers) or 3 weeks (Lascar US customers) to calibrate this product, please place your order today and our sales team will then confirm the exact delivery date.
Click here to view a sample certificate.
The EL-21CFR-VAC temperature data logger has been specifically developed to meet the data security required by the FDA’s 21CFR part 11 regulations. As such it is suitable for use in applications where the integrity of the data is of the highest importance.
For application stories on our data loggers, visit our case studies section.
Vaccine Storage
Whether you’re optimizing processes, safeguarding stock, or storing and transporting valuable assets, reliable data is critical to success.
Pharmaceutical Cold Chain
Using precise temperature loggers in pharmaceutical cold chains to ensure drug efficacy, quality, and health standard compliance.
21CFR Compliant Data Loggers
Offering 21CFR compliant data loggers ensuring secure, reliable data management and regulatory compliance.
Blood Storage
Implementing advanced data loggers for precise blood storage monitoring, ensuring safety and health standard compliance.
21CFR Part 11 is an American FDA regulation covering electronic data records for food and pharmaceutical manufacturers. 21CFR-compliant systems require our 21CFR designated hardware and our separate 21CFR cloud service.
An administrator for the software will need to reset your password from the <Admin> <Edit User> menu. The next time you attempt to log in it will ask you to enter and confirm a new password. For further guidance on software use, please reference the
21CFR Software Guide.More Support